GenoType MRSA – The basic test for culture confirmation
Your test system for rapid identification of methicillin-resistant Staphylococci from culture samples
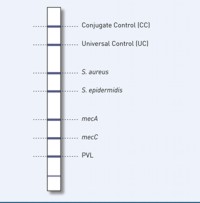 Our GenoType MRSA test system permits molecular genetic identification of S. aureus and S. epidermidis from cultures. Differentiation of the clinically most significant pathogens causing staphyloccocal infections is thus assured. Since S. aureus exhibits particularly high pathogenicity, its reliable detection is crucial for the introduction of targeted treatment and hygiene measures. In addition, the mecA and mecC genes that impart methicillin resistance as well as a specific fragment of the PVL gene are simultaneously detected with the GenoType MRSA . The presence of PVL gives an indication of CA-MRSA strains acquired in the environment. In contrast to nosocomial MRSA, CA-MRSA have a higher pathogenicity, but do not demonstrate any multiresistance to antibiotics. Distinguishing CA-MRSA from nosocomial MRSA therefore makes sense from a therapeutic and epidemiological viewpoint.
Our GenoType MRSA test system permits molecular genetic identification of S. aureus and S. epidermidis from cultures. Differentiation of the clinically most significant pathogens causing staphyloccocal infections is thus assured. Since S. aureus exhibits particularly high pathogenicity, its reliable detection is crucial for the introduction of targeted treatment and hygiene measures. In addition, the mecA and mecC genes that impart methicillin resistance as well as a specific fragment of the PVL gene are simultaneously detected with the GenoType MRSA . The presence of PVL gives an indication of CA-MRSA strains acquired in the environment. In contrast to nosocomial MRSA, CA-MRSA have a higher pathogenicity, but do not demonstrate any multiresistance to antibiotics. Distinguishing CA-MRSA from nosocomial MRSA therefore makes sense from a therapeutic and epidemiological viewpoint.
The test is thus as an optimal basic test for culture confirmation the foundation for cost-effective MRSA diagnostics.
Your benefits with GenoType MRSA
- Differentiability: Detecting S. aureus and S. epidermidis with only one test offers you the basis for a safe therapy and hygienic management.
- High specificity: Simultaneous detection of the resistance mediating genes mecA and mecC as well as a PVL-specific fragment provide unambiguous results and reliable MRSA identification. A distinction between CA-MRSA and nosocomial infections is thus provided.
- Rapid result: Performing the test from the overnight culture provides you with a result in only 4 hours and thus guarantees rapid diagnosis.
Not all of our products are available in every country. Please contact your local sales representative for availability of this IVD product in your country.
At a glance
Molecular genetic assay for the fast identification of Methicillin-Resistant Staphylococci from cultured material
Starting material:
freshly grown bacteria
DNA Isolation:
methods producing amplifiable DNA from bacteria (for example QIAamp DNA Mini Kit from Qiagen) or quick protocol (for further informaton please refer to the manual)
Order number:
- 12 tests No. 30512
DNA•STRIP technology
Downloads:
Information request:
More Information:
