GenoType MTBDRplus VER 2.0 - Your Test System for a Fast and Reliable Way to detect MDR-TB
Identification of the M. tuberculosis complex and its resistance to Rifampicin and/or Isoniazid from pulmonary clinical specimens or cultivated samples
The emergence and spread of multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) are a major medical and public problem threatening the global health.
MDR-TB is caused by mycobacteria which are at least resistant to the two most powerful first-line anti-TB drugs rifampicin and isoniazid. Conventional methods for mycobacteria culture and drug susceptibility testing are slow and elaborate, requiring sequential procedures for the diagnosis. During this time patients may be treated inappropriately, drug resistant strains may continue to spread, and amplification of resistance may occur. Therefore rapid diagnosis and identification of MDR-TB strains are prerequisites for the worldwide fight against TB.
The GenoType MTBDRplus enables a rapid result from pulmonary patient specimen and from culture material. Also for diagnosing patients after treatment failure and relapse, with unknown anamnesis and originating from high prevalence areas of MDR-TB as well as for diagnosing patients in high prevalence TB countries and high burden MDR-TB regions the use of GenoType MTBDRplus is reasonable. Finally the test can also be applied for screening purposes to develop country-specific TB action plans.
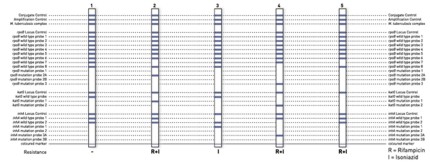
The identification of rifampicin resistance is enabled by the detection of the most significant mutations of the rpoB gene (coding for the β-subunit of the RNA polymerase). For testing the high level isoniazid resistance, the katG gene (coding for the catalase peroxidase) is examined and for testing the low level isoniazid resistance, the promoter region of the inhA gene (coding for the NADH enoyl ACP reductase) is analyzed.
Your benefits with GenoType MTBDRplus
- Can be performed from pulmonary patient specimen and from culture material.
- Results are obtained in 5hrs compared to 1 to 2 months with conventional methods.
- Allows early, appropriate treatment, which reduces transmission and spread of MDR-TB.
Not all of our products are available in every country. Please contact your local sales representative for availability of this IVD product in your country.
At a glance
Molecular genetic assay for identification of resistance to rifampicin and/or isoniazid of Mycobacterium tuberculosis complex
Starting material:
Pulmonary specimens and/or liquid/solid culture samples
DNA Isolation:
GXT DNA/RNA Extraction Kit (with GenoXtract®) or
manually with GenoLyse®
Order number:
- 12 tests No. 304A
- 96 tests No. 30496A
DNA•STRIP technology
Downloads:
Information request:
More Information: