FluoroType® Parvovirus B19
Your molecular genetic test system for quantitative detection of parvovirus B19
Parvovirus B19 is spread worldwide with a seroprevalence of 60 - 90% in adults. The primary route of transmission is via respiratory droplets, but blood-borne transmission is also possible. Parvovirus B19 interferes with production of red blood cells by mediating the destruction of their progenitor cells in the bone marrow. Primary infection most commonly occurs in children, causing rather mild symptoms or manifesting as erythema infectiosum (fifth disease), whereas it may cause arthropathy in adults. However, infection during pregnancy is very dangerous, as it can lead to severe or even fatal fetal complications, such as hydrops fetalis. Parvovirus B19 infection is particularly dangerous for patients with preexisting hemolytic diseases or for immunocompromised patients (e.g. after organ transplantation), in which the virus causes either acute or chronic anemia.
PCR-based assays are an effective tool to detect highly acute parvovirus B19 infections, even in immunocompromised patients, where infections might be overlooked by serological assays. Further diagnostic steps or suitable therapeutic measures can thus be met at an early stage.
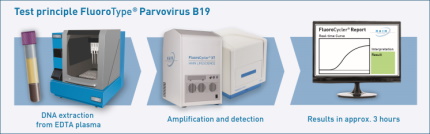
Viral DNA is extracted automatically from EDTA plasma with the GenoXtract® followed by amplification, detection and quantification of characteristic target sequences with real-time PCR using the FluoroCycler® XT instrument. Internal controls monitor test performance from sample preparation to test result. Reliable viral load assessment in International Units (IU/ml according to NIBSC WHO International Standard) requires only two quantification standards, which are determined just once per kit lot, eliminating the need for standard measurements with each run. Additionally, virus concentrations beyond linear quantification range are evaluated as qualitative results. Interpretation of results is performed automatically by the FluoroSoftware®, for reliable test results within only three hours.
Your benefits of using FluoroType® Parvovirus B19
- Reliable quantification: Viral load assessment in IU/ml with two quantification standards, which are determined only once per kit lot. Virus concentrations beyond the limit of quantification are evaluated as qualitative results.
- User-friendly: Automated DNA extraction for minimal hands-on time and an efficient workflow. Interpretation of results is performed automatically by the FluoroSoftware®.
- Fast and dependable results: Internal controls monitor test performance from sample preparation to amplification and detection for reliable results within only three hours.
- Maximum flexibility: Combination with further viral parameters of our portfolio due to a universal test procedure. Single samples as well as high sample numbers can be analyzed efficiently according to your needs.
- CE-marked: No need for elaborate validation studies.
Not all of our products are available in every country. Please contact your local sales representative for availability of this IVD product in your country.
At a glance
Molecular genetic test system for quantitative detection of parvovirus B19
Starting material:
EDTA plasma
DNA extraction:
GXT NA Extraction Kit (with GenoXtract®)
Instrument for aplification and detection:
FluoroCycler® XT
Order Numbers:
- 24 Tests No. 62224
- GXT NA Extraction Kit No. 12.08.02
FluoroType® technology
Downloads:
Information request: