GenoType NTM-DR VER 1.0
Your molecular genetic test system for the detection of resistance to macrolides and aminoglycosides in various clinically relevant NTM
The incidence of infections caused by nontuberculous mycobacteria (NTM) is increasing since years and therefore adequate therapy regimens are becoming more and more important. Within the group of NTMs, especially the members of the M. avium and M. abscessus complex are in the focus of interest. Infections caused by those mycobacteria are difficult to treat due to drug resistances. Thus, the treatment outcome differs significantly.
Macrolides are an important component of the antimycobacterial drug therapy. However, resistances to macrolides may limit treatment options and endanger therapeutic success. One potential resistance mechanism is mediated by mutations within the rrl gene. Another mechanism that only affects the members of the M. abscessus complex is mediated by the erm(41) gene. This gene influences susceptibility against clarithromycin of the subspecies - therefore the detection of erm(41) should be part of molecular genetic resistance testing.
Aminoglycosides are also often used to treat NTM diseases. Resistance to aminoglycosides is primarily caused by mutations in the rrs gene.
Consequently, the management of NTM infections requires the rapid determination of resistance status in order to provide an appropriate therapy.
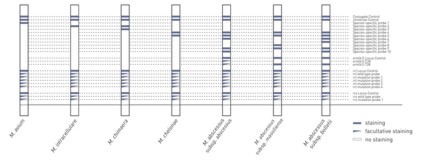
GenoType NTM-DR allows the detection of several clinically relevant NTMs including the differentiation between M. intracellulare and M. chimaera. Furthermore, the mycobacteria resistance to macrolides and aminoglycosides are also detected within the same step. Thus, GenoType NTM-DR provides crucial information as a prerequisite for an adequate therapy regimen.
Your benefits with GenoType NTM-DR
- Reliable results: GenoType NTM-DR allows the simultaneous detection and differentiation of several nontuberculous mycobacteria (NTM) and their resistance to macrolides and aminogycosides from cultured material. Thus, this test system is an additional tool for the diagnosis and treatment regimen of NTMs.
- Striking information: The test can uniquely differentiate between M. intracellulare and M. chimaera using a proven technology.
- Rapid results: Results with detailed information are available within 5 hours only compared to laborious and time-consuming conventional methods.
- User-friendly: The well-established DNA•STRIP technology enables manual or automated processing - thus a convenient implementation in your lab is easily possible.
- CE-marked: No need for elaborates validation studies.
Not all of our products are available in every country. Please contact your local sales representative for availability of this IVD product in your country.
At a glance
Molecular genetic assay for detection of resistance to macrolides and aminoglycosides in various nontuberculous mycobacterial Species (NTM)
Starting material:
Bacteria grown on solid medium or in liquid medium
DNA Isolation:
GenoLyse®
Order number:
- 12 tests No. 29712
DNA•STRIP technology
Downloads:
Information request: