FluoroType® STI – powered by LiquidArray®
Raising awareness for STI detection
According to the WHO, more than 1 million sexually transmitted infections (STIs) are acquired every day, the majority of which are asymptomatic (1). Undiagnosed, these infections can have a significant impact on the long-term health and wellbeing of infected individuals, which subsequently increases the burden on healthcare systems. Reliable diagnostics therefore are a crucial tool for managing STIs and their impact.
FluoroType® STI
FluoroType® STI is a multiplex panel PCR test for detection of 9 targets covering 7 major sexually transmitted pathogens in a single test – powered by the pioneering LiquidArray® technology. The most common bacterial sexually transmitted infections (STIs) are caused by Chlamydia trachomatis and Neisseria gonorrhoeae. In addition, Trichomonas vaginalis and Mycoplasma genitalium are considered “important and under-recognised” causes of STI. The detection of three further STI-related species (Mycoplasma hominis, Ureaplasma parvum and Ureaplasma urealyticum) complement an essential and reliable testing.
The test is validated for use with genital swabs and urine; the kit contains reagents for up to 48 reactions. PCR results are produced in under two hours.
Neisseria gonorrhoeae
The accurate diagnosis of N. gonorrhoeae presents a particular diagnostic challenge. This is due to high recombination rates between different Neisseria species, and non-pathogenic Neisseria species colonizing the pharynx. FluoroType® STI provides confidence in N. gonorrhoeae reporting by requiring the detection of two independent genes to return a N. gonorrhoeae positive result. When only a single gene is detected a Neisseria species result is returned, which can be further investigated, if desired, to determine whether treatment is required.
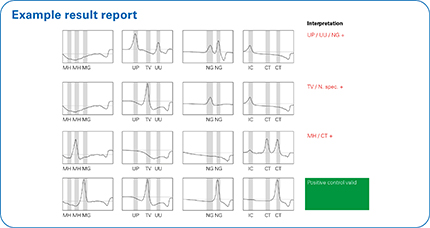
Results at a glance in combination with FluoroCycler® XT
When used in combination with the FluoroCycler® XT, the integrated FluoroSoftware® XT-IVD produces an unambiguous report that can be read ‘at a glance’. The wizard-based software guides users easily through setup and results evaluation. The Internal Control and software assisted evaluation ensures the generation of reliable results.
LiquidArray®
The pioneering LiquidArray® technology provides next generation multiplexing capabilities. The method optimizes asymmetrical multiplex PCR for creating excess single-stranded amplicons with detection by Lights-On/-Off probes that contain a quencher (Lights-Off) or both fluorophore and quencher (Lights-On). If both probes are hybridized in proximity on the amplicon, the quencher of the Lights-Off probe eliminates the fluorescence emitted by the Lights-On probe. During melting curve analysis, Lights-On/-Off probes detach from the amplicon at specific temperatures. In the unbound state, Lights-On probes cannot emit, and as fluorescence is either emitted or suppressed, specific fluorescence signatures are generated by the unique FluoroCycler® XT thermocycler for the LiquidArray® technology.
Automated nucleic acid extraction for optimal performance
The GenoXtract® nucleic acid extraction systems, in combination with dedicated extraction kits, provide high quality nucleic acid extraction for optimal performance.
GenoXtract® allows flexible processing of 1 – 12 samples with ready-to-use reagent cartridges and consumables in a low to medium throughput setting. Due to secure sample processing and unique disposable pipetting system, which minimize the potential for contamination, users can process samples with confidence. To support medium throughput workflows, multiple instruments can be installed in parallel.
FluoroType® STI is also validated with other extraction option. Contact us for more details.
Your benefits with FluoroType® STI
- Detection of up to 7 of the most prevalent bacterial and parasitic pathogens: In addition to the most common pathogens, Chlamydia trachomatis and Neisseria gonorrhoeae, the assay also detects Trichomonas vaginalis, 2 different Mycoplasma and 2 Ureaplasma species. This target selections creates an essential STI panel powered by the next generation multiplexing LiquidArray® technology.
- Confidence in Neisseria gonorrhoeae reporting: FluoroType® STI requires the detection of two independent genes to return a N. gonorrhoeae positive result. When only a single gene is detected a Neisseria species result is returned, which can be further investigated, if desired, to determine whether treatment is required.
- Combination with FluoroCycler® XT provides ‘results at a glance’: Automatically generated reports allow for fast, confident reporting without the need for individual interpretation.
- Streamlined workflow from sample to result: The combination of FluoroType® STI, FluoroCycler® XT and GenoXtract® provides a fully validated workflow for ease of implementation in your laboratory.
Please contact your local representative for availability in your country. Not for sale in the USA.
1) World Health Organization. (2019). Sexually transmitted infections: evidence brief. World Health Organization. https://apps.who.int/iris/handle/10665/329888. License: CC BY-NC-SA 3.0 IG
At a glance:
Multiplex syndromic panel PCR assay for qualitative detection of 7 sexually transmitted pathogens directly from clinical specimens.
Number of reactions:
48
Sample material:
genital swabs and urine
RNA extraction:
GXT NA Extraction Kit (with GenoXtract®)
Instruments for amplification and detection:
Order numbers:
FluoroType® STI No. 1877362
GXT NA Extraction Kit No. 12.08.02 (96 samples)
Technology:LiquidArray®
Downloads:
Information request: