FluoroType® SARS-CoV-2 plus
Coronavirus Disease 2019 (COVID-19)
In 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged as a novel pathogenic virus causing coronavirus disease (COVID-19). The emerging COVID-19 crisis has been categorised as a pandemic, according to the World Health Organization.
While most cases present with mild or moderate symptoms, the older population and those with underlying health conditions are susceptible to severe symptoms and increased mortality.
As healthcare systems across the world battle this urgent healthcare problem, rapid detection of COVID-19 is essential for outbreak management to help mitigate pressures on healthcare services.
FluoroType® SARS-CoV-2 plus
FluoroType® SARS-CoV-2 plus is a multiplex real-time PCR test, which targets and differentiates two SARS-CoV-2 genes, while simultaneously detecting endemic human coronaviruses (HCoV).
The kit is validated on respiratory samples (nasopharyngeal and oropharyngeal swabs) for up to 96 results produced in under two hours. Internal Controls (Universal Internal Control 2, available as a separate product) monitor extraction, reverse-transcription and amplification for maximum confidence.
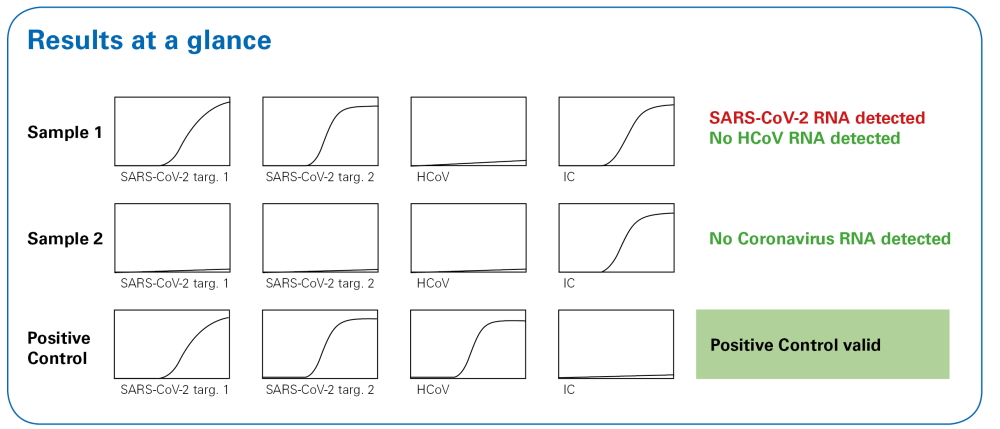
Results at a glance in combination with FluoroCycler® XT
When used in combination with the FluoroCycler® XT, the integrated FluoroSoftware® XT-IVD produces an unambiguous report that can be read ‘at a glance’. The wizard-based software comfortably guides users through setup and results evaluation. The Internal Control and software assisted evaluation ensures the generation of reliable results.
FluoroType® SARS-CoV-2 plus is also validated on common laboratory thermocyclers. Contact us for more details.
Automated nucleic acid extraction for optimal performance
FluoroType® SARS-CoV-2 plus is also validated with other extraction options. Contact us for more details.
Your benefits with FluoroType® SARS-CoV-2 plus
-
Detection of SARS-CoV-2 with two independent targets
Two independent genes of the SARS-CoV-2 genome are targeted, providing confident identification of SARS-CoV-2. -
Differentiation from endemic human coronaviruses
Four endemic human coronaviruses (HCoV-NL63, HCoV-OC43, HCoV-229E, HCoV-HKU1), are detected in a separate channel, allowing differentiation from SARS-CoV-2. -
Combination with FluoroCycler® XT provides ‘results at a glance’
Automatically generated reports allow for fast, confident reporting without the need for individual interpretation. -
Validated workflow supports ease of laboratory implementation
The combination of FluoroType® SARS-CoV-2 plus, FluoroCycler® XT and GenoXtract® with dedicated GXT NA Extraction Kit provides a fully validated workflow for ease of implementation in your laboratory. -
Other instruments are validated to maximise flexibility for the laboratory
Due to the scale of demand in laboratories, the kit is also validated on third party systems.
The performance characteristics of FluoroType® SARS-CoV-2 plus and FluoroType® SARS-CoV-2/Flu/RSV are unaffected by the mutations of the recently described SARS-CoV-2 strain VOC-202012/01 (or lineage B.1.1.7 or 20B/501Y.V1) emerging from the United Kingdom as well as the South African 501.V2 variant (also known as 501.V2, 20C/501Y.V2 or B.1.351 lineage). Sequence comparison via in silico analysis showed no influence of the reported mutations on the reliability for detection of the described SARS-CoV-2 variants by both FluoroType® assays.
Not all of our products are available in every country. Please contact your local sales representative for availability of this IVD product in your country.
At a glance
Real-time PCR assay for specific detection of SARS-CoV-2 and endemic HCoV
Number of reactions:
96
Sample material:
nasopharyngeal and oropharyngeal swabs
RNA extraction:
GXT NA Extraction Kit (with GenoXtract®)
GXT96 X3 ExtractionKit (with GenoXtract® fleXT)
Instruments for amplification and detection:
FluoroCycler® XT
Order number:
- FluoroType® SARS-CoV-2 plus No. 1877361
- Universal Internal Control 2 No. 402960
- GXT NA Extraction Kit No. 12.08.02
-
GXT96 X3 Extraction Kit No. 550960
FluoroType® technology
Downloads:
Information request: