FluoroType® technology - innovative technology with highest sensitivity and maximum specificity
Our fluorescence-based PCR test systems guarantee rapid and valid results for efficient diagnostics in your laboratory. Nucleic acids are extracted from sample materials in a first step, followed by amplification and detection using fluorescence-labeled probes. Amplification and detection are carried out with the FluoroCycler®. The FluoroType® technology is based on different methods:
PCR with hybridization probes
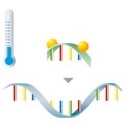  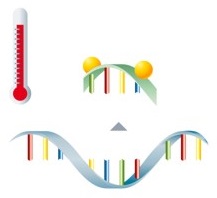 |
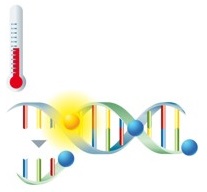
Detection via fluorescence-labeled probes takes place directly after amplification of the target sequences. The probes are marked with fluorophores and are complementary to the amplified nucleic acids. The fluorophore can't be excited to emit fluorescence if the probes are unbound.
During the subsequent melting curve analysis, the probes dissociate from the amplicon at their specific temperature, leading to an immediate decrease in fluorescence. |
PCR with hydrolysis probes
 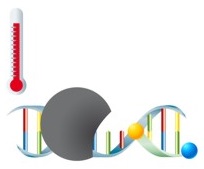 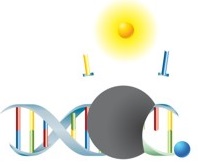 |
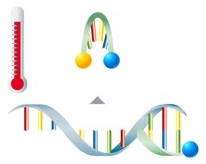
With this variant of real-time PCR, detection of the amplicon is performed using hydrolysis probes. The probes are labeled with a fluorophore and a quencher. In the unbound state, no fluorescence emission occurs, since both molecules - fluorophore and quencher - are in close proximity. During amplification, the probes hybridize with the target sequences and are hydrolytically degraded by the DNA polymerase during the elongation phase of the PCR. Fluorophore and quencher of the degraded probes are released into the medium and therefore are no longer in close proximity – the fluorescence signal can now be detected. The increase in fluorescence intensity can thereby be tracked in real-time in parallel to the increase of free fluorophore. Moreover, this technology offers the possibility of quantification of the target sequences. |
LiquidArray® - Asymmetric excess PCR with Lights-On/-Off-Probes
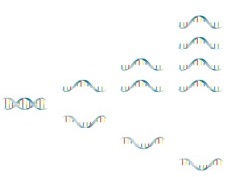    |
The innovative LiquidArray® technology is an optimized asymmetrical PCR, where one of the primers is present in excess. The second primer is only present in a limited concentration. Because of this limitation, a linear increase of single-stranded nucleic acid occurs after a short exponential phase (with both primers in action) – thus creating excess single-stranded amplicons. Afterwards the detection is carried out using Lights-On/-Off probes.
|