GenoType LepraeDR VER 1.0
Your test system for the identification of Mycobacterium leprae and its resistance to rifampicin, ofloxacin and dapsone
Leprosy, also known as Hansen’s disease, is a chronic infectious disease, caused by Mycobacterium leprae, an acid-fast, rod-shaped bacillus. The bacilli affect skin, mucous membranes, peripheral nerves, eyes and internal organs, in some cases the lymph nodes. The incubation time of the disease can take up to 20 years and more. On average it takes 2-4 years for the symptoms to appear.
Leprosy occurs in several clinical manifestations. In general it is classified in tuberculoid leprosy (“paucibacillary”) and lepromatous leprosy (“multibacillary”). There is a wide spectrum of intermediate forms, also referred to as borderline leprosy.
Even if leprosy is thought to be eradicated in most of the countries, according to the WHO, we still had to face about 213.000 new cases worldwide at the beginning of 2009. An estimated 3 million people are suffering from long-term consequences. Until now preventative measures in form of immunization are not available.
The bacilli have never been cultured in vitro. The common diagnostic method is the detection of Mycobacterium leprae in skin smears. The microscopy allows the differentiation between paucibacillary (PB) leprosy (= smear-negative) and multibacillary (MB) leprosy (= smear-positive), which is an important information with regard to therapy.
Because experience in practical application of histology and/or molecular biology is required, these sensitive detection methods are mainly performed in reference laboratories. The same also applies for the drug susceptibility testing in mouse foot pads which is very labour- and time-consuming. On the basis of these facts, it is difficult to get reliable data about primary and secondary antibiotic resistance rates; thereby resistances to rifampicin and dapsone are described.
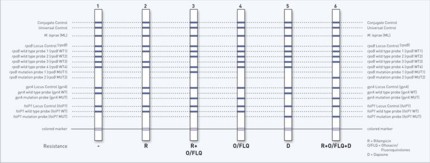
Now, a fast and reliable test system for the molecular genetic identification of M. leprae and its antibiotic resistances is available: The GenoType LepraeDR permits the simultaneous detection of M. leprae and its resistance to rifampicin, ofloxacin and dapsone within a few hours.
Your benefits with GenoType LepraeDR
- First commercial test system for the molecular detection of Mycobacterium leprae.
- Results are obtained in 5 hours compared to several months with conventional methods.
- Detection of resistance to first- and second-line drugs in one step.
- Safe and reliable due to internal controls and the combination of specific amplification and hybridization.
- Allows early, appropriate treatment which reduces long-term consequences.
Not all of our products are available in every country. Please contact your local sales representative for availability of this IVD product in your country.
At a glance
Molecular genetic assay for identification of resistance to rifampicin, ofloxacin, and dapsone of Mycobacterium leprae
Starting material:
AFB(acid-fast bacilli)-positive skin biopsies
DNA Isolation:
Quick protocol (for further informaton please refer to the manual)
Order number:
- 12 tests No. 320
DNA•STRIP technology
Downloads:
Information request:
More Information: